What Is Nuclear Magnetic Resonance Spectroscopy (NMR)?
NMR spectroscopy is an analytical technique which detects the magnetic spin of certain atomic nuclei and uses that to elucidate molecular structures and dynamics. When a nuclei with a non-zero spin value (I≠0) is placed in a static magnetic field, the nuclei will process at a characteristic resonance frequency. This frequency depends on static field strength, the chemical environment and the isotope type. A simple one-dimension (1D) NMR data is a plot of frequency/chemical shift vs intensity.
NMR shows strong capabilities in elucidating complex molecular structures, especially of organic materials. Functional groups, bond connectivity, stereochemistry and spatial configuration can all be revealed by various NMR methods. It is also non-destructive where the samples are typically analyzed as dissolved solutions or in solid state. Materials are not exposed to high energy irradiation (e.g. X-ray, electron beam, ion beam, plasma etc.) and can be easily recovered. NMR is quantitative where the signal intensity is directly proportional to the number of nuclei contributing to that signal. This allows NMR to be an excellent quantification method for assessing material purity, monitoring reaction kinetics etc.
Definitive Structural Clarity
Determines atomic connectivity, stereochemistry, and spatial configuration with unmatched precision.
Versatile Sample Compatibility
Works on solutions, solids, mixtures, and macromolecules, across organics, polymers, biomolecules, and inorganics.
Quantitative and Non‑Destructive
Provides absolute quantification while preserving the integrity of valuable or limited samples.
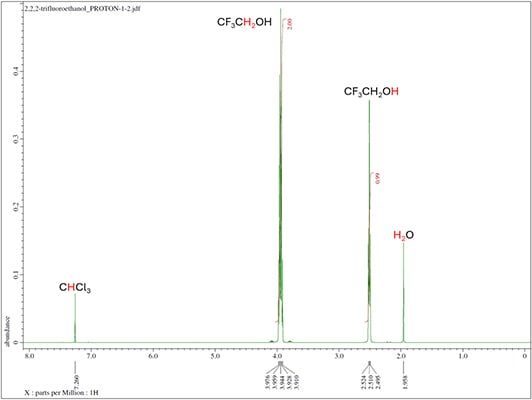
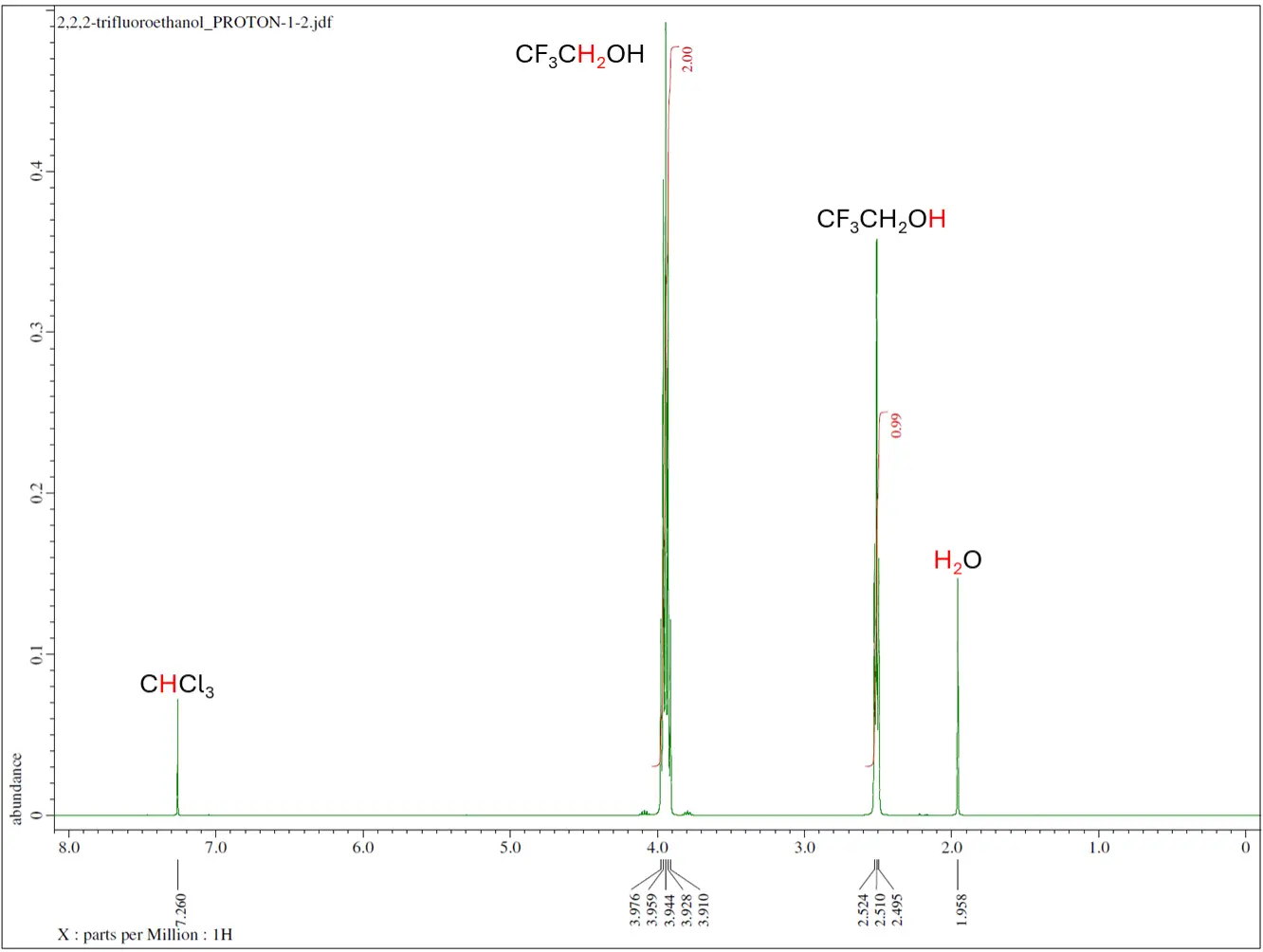
- Proton NMR collects the resonance signals from protons in the sample.
- Proton exhibits high NMR sensitivity due to its high natural abundance (99.985%) and large gyromagnetic ratio.
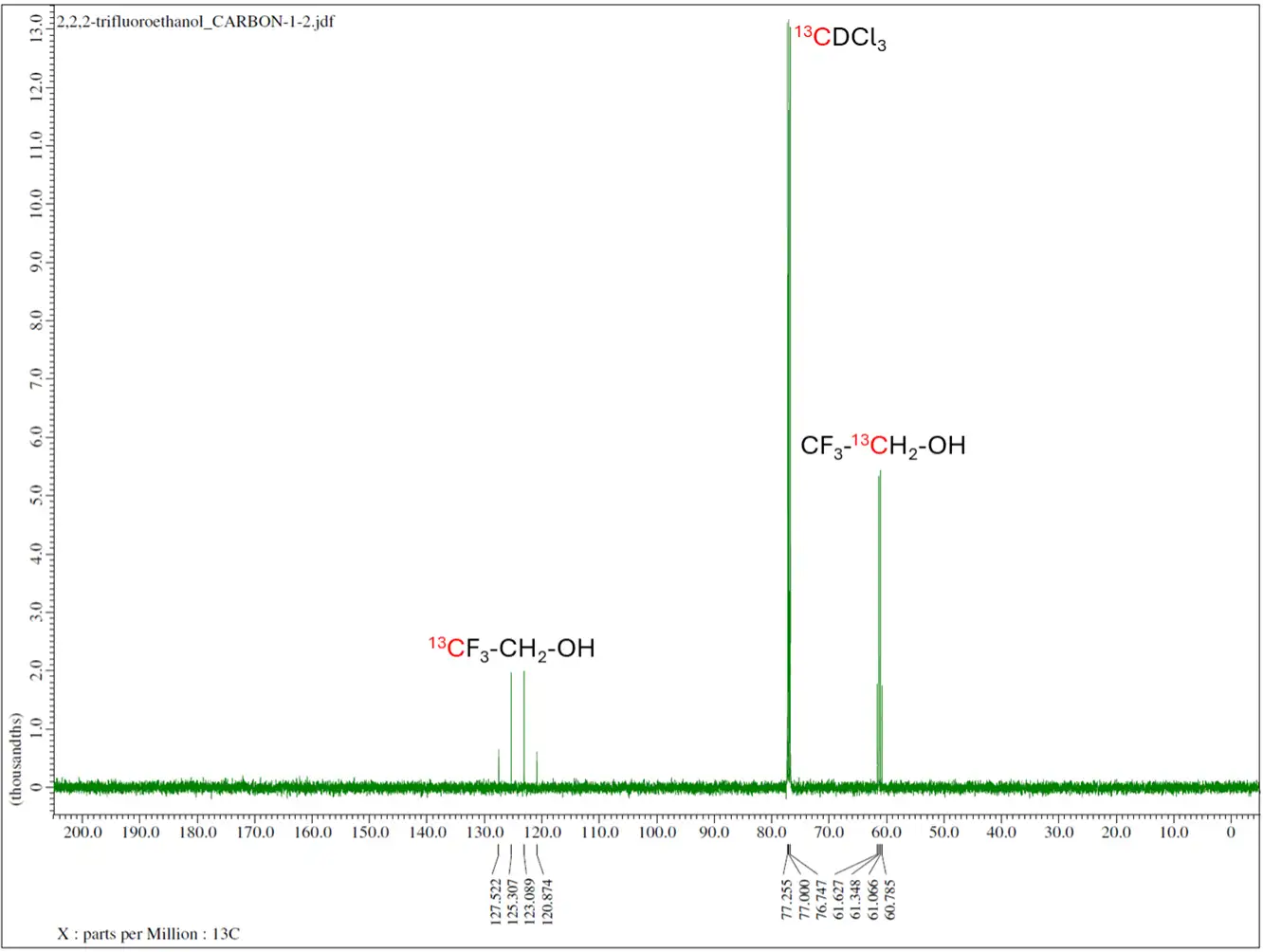
- 13C NMR collects the carbon-13 NMR signals from the sample. Carbon-12 is the most abundant carbon in nature; however, it’s NMR inactive due to a spin value (I) of 0.
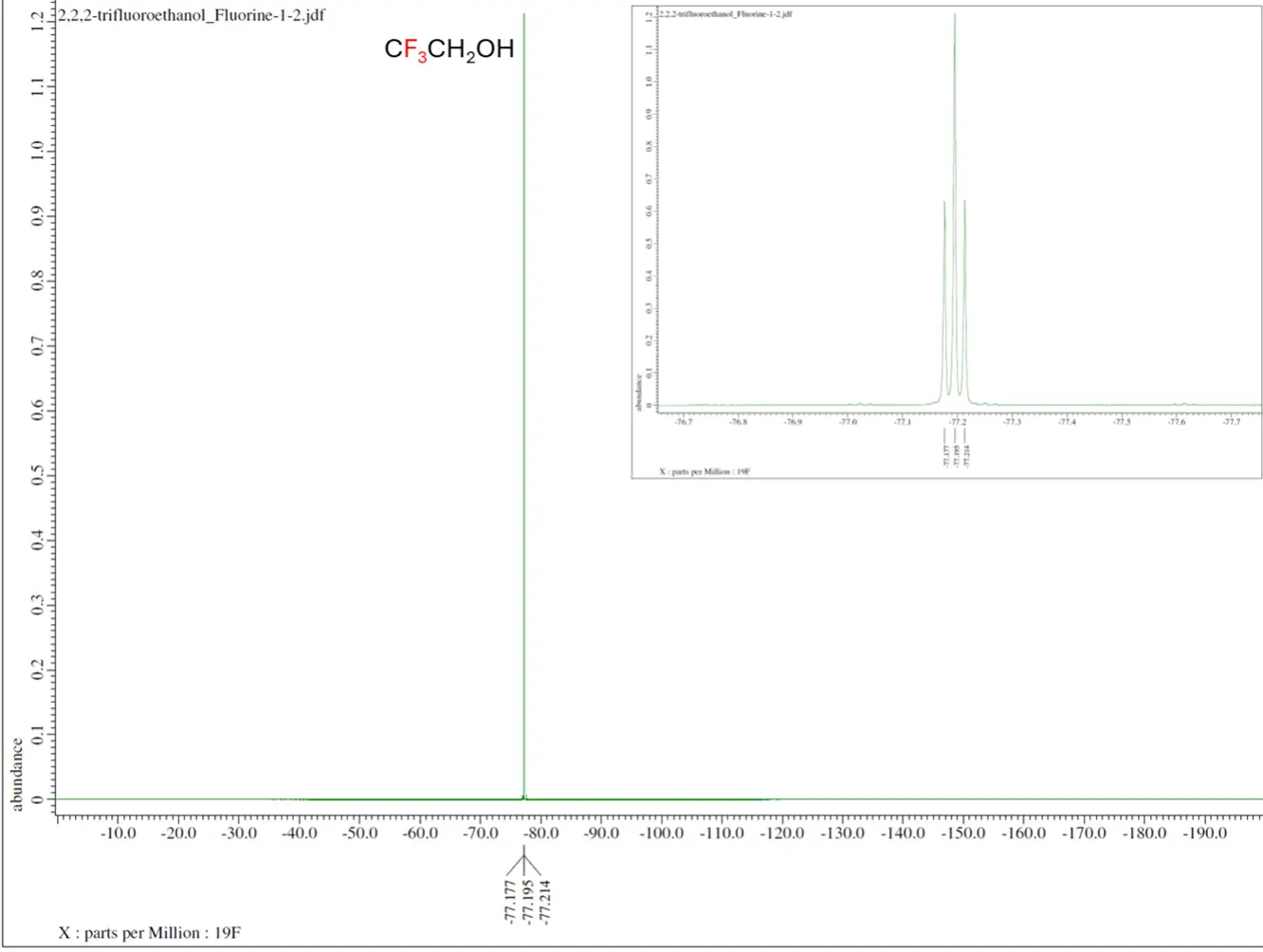
- Multi-nuclear NMR refers to detection of NMR-active isotopes other than the common 1H and 13C. This includes 6/7Li, 10/11B, 15N, 19F, 27Al, 29Si, 31P etc.
Why Use NMR?
- Are complimentary techniques to FTIR, GCMS and LCMS for analyzing organic materials.
- Can be used instead of XRD for analyzing inorganic materials when no diffraction patterns are observed.
- Can be used instead of GCMS or LCMS for quantification when the reference material is not available, because NMR is a primary quantification method which doesn’t need a calibration curve. Solid NMR can be used instead of GCMS or LCMS when no destruction on the solid sample is required or the sample is insoluble in any solvent.
Complementary Strength
Fills structural detail gaps when FTIR, GC‑MS, LC‑MS, or XRD fall short.
Calibration‑Free Quantification
Acts as a primary method for absolute concentration without external standards.
Solid + Liquid Flexibility
Applicable to both soluble samples and solid materials, enabling broader problem‑solving.
Working Principle
NMR uses a strong static magnetic field and a weak oscillating field to probe the magnetic properties of the selected atomic nuclei in a sample. As the nuclei undergo spin-state polarization and relaxation, the resulting signals reflect their electromagnetic environments, revealing molecular structure and composition. Repeated polarization and varying field strengths enable 1D and 2D analyses, allowing for quantitative structure determination and monitoring of dynamic changes. NMR is typically performed on liquids, but solid-state and low-temperature variations exist.
When placed in a static magnetic field, nuclei process at a resonance frequency, detected by applying a radio frequency pulse and measuring the emitted signal during relaxation. This signal (called free induction decay) is Fourier transformed to produce a frequency spectrum, usually converted to chemical shift (δ, ppm) using a reference compound like tetramethylsilane (TMS). NMR also reveals spin coupling, where signals split into multiplets based on neighboring nuclei, with the coupling constant (J) indicating bond distance and configuration. Advanced 2D NMR uses composite pulse sequences to correlate nuclei and elucidate molecular structures. Spectral resolution improves with higher magnetic field strength, resulting in sharper peaks.
Equipment Used for NMR:
JEOL ECZL-G 500MHz NMR spectrometer
- Multi Frequency Drive System enables triple‑resonance experiments (HCN/HCX/HCP) on a 2‑channel console.
- 5 ns sequencing, phase‑modulated pulses (e.g., PM‑BEBOP) for wide‑band nuclei like 19F.
- 16‑bit 100 Msps Digital Quadrature Detection and low‑noise SiGe preamps for high dynamic range and improved sensitivity.
- Digital lock with fast and accurate feedback allows stability for long time measurements.
- Rapid automated 1D and 3D gradient shimming (including on 1H, 2H, 19F).
Integrated Accessories / Probes:
- ROYALPROBETM HFX: 1H/19F dual‑tune capability for HFX experiments and clean spectra of fluorinated compounds.
- SuperCOOL cryogenic probes: Cryo‑cooled detection coil and preamplifier for multi‑fold sensitivity gains over room‑temperature probes.
- GR5AT Diffusion Probe: High‑power PFG for DOSY/diffusion, polymers, and low‑frequency nuclei studies.
- MiddleRotor HXMAS: Solid‑state MAS with balanced volume/spin speed; ideal for 13C in organics.
- Solid‑State Triple‑Resonance MAS (HXY/HCN): Enables 1H/13C/15N and 31P/27Al correlations for biosolids, zeolites, catalysts.
- Auto Sample Changer (ASC30/64/100): Safe, flexible handling of tube types/sizes.

Key Differentiators
Covalent possesses both liquid and solid-state NMR probes. For liquid NMR, we have a standard probe for routine 1D and 2D NMR work. This probe can be either single-tuned (1H or 19F) or dual-tuned (1H+19F) on the high frequency channel, which makes it excellent in characterizing complex fluorinated materials for polymer and pharmaceutical industry. We also have a cryogenic-cooled liquid probe for higher sensitivity work (limited sample size, low natural abundance isotopes) and a strong gradient probe for more demanding PFG NMR work. For solid NMR, we have a two-channel (HX) and a three-channel (HXY) Magic-Angle-Spinning (MAS) NMR probe for 1D, 2D solid NMR work which is our specialty and is not commonly available in industrial labs.
Strengths
- Unambiguous Structural Information: For determining the precise atomic arrangement and connectivity, NMR is often the definitive technique, providing information that fragmentation-based methods like mass spectrometry cannot.
- Analysis of Intact Molecules: NMR analyzes molecules in their native state, often in solution, without the need for derivatization or fragmentation, which can alter the compound.
- Non-Destructive Nature: Samples can typically be recovered after NMR analysis, which is crucial for valuable or limited materials.
- Quantitative Accuracy: qNMR provides reliable quantitative data without the need for external calibration curves for each analyte in a mixture.
- Versatility: NMR can be applied to a wide range of samples, from small organic molecules to large biomolecules and even solid materials (with solid-state NMR).
Limitations
- Low sensitivity isotopes require larger sample amounts (milligrams) and higher concentrations (millimolar) for a good signal-to-noise ratio.
- High-resolution liquid NMR needs samples to be dissolved in deuterated solvents; insoluble materials require solid-state NMR.
- Complex mixtures cause overlapping signals, complicating interpretation; paramagnetic impurities reduce resolution.
- Sample homogeneity is crucial; solids, multiple phases, or viscous samples broaden peaks and degrade quality.
- Low sensitivity nuclei like 13C and 15N demands longer acquisition or isotopic enrichment.
- Complex spectra from large molecules or mixtures can be crowded, hindering complete assignment.
- Large molecules have broadened signals due to slow tumbling, reducing resolution and sensitivity.
- NMR only detects nuclei with non-zero spin; common elements like 12C and 16O are invisible.

Unsure Whether NMR Is Right for You?
Validate structure, purity, and dynamics with expert 1D/2D and solid‑state NMR guidance.
Sample Information
What we accept:
- For liquid NMR, samples must be fully soluble in at least one deuterated solvent with no solid particles or paramagnetic materials present, such as iron oxide. A minimum of 0.7 mL is required if already dissolved, and recommended sample amounts are 5–10 mg for ^1H or ^19F, and 50–100 mg for ^13C or ^31P analyses. Standard deuterated solvents (CDCl₃, D₂O, DMSO-d₆, CD₃CN) are provided, with special solvents or TMS available upon request; consult NMR staff for oxygen- or moisture-sensitive or corrosive samples.
- For solid-state NMR, samples should be fine particles or chunks that can be ground, must be air stable, and require a minimum of 15 mg, with corrosive solids not accepted.
Use Cases

Pharmaceuticals
Determining chemical structures of drug candidates, analyzing solid-state properties and drug-excipient interactions, confirming identity and purity for QC, characterizing biologics, and monitoring drug release.

Chemical Industry
Identifying molecular structures, measuring purity and quantifying mixtures, monitoring reactions in real-time, and ensuring product quality.

Materials Science & Polymers
Characterizing polymer composition and structure, analyzing materials like composites and ceramics, studying catalysts and porosity, and monitoring material degradation.

Food & Agriculture
Measuring key components for QC, verifying product authenticity, monitoring processes like fermentation, and analyzing fatty acid profiles.

Energy (Oil, Gas and Batteries)
Analyzing rock cores and crude oil, characterizing battery and fuel cell materials, studying ion transport, and monitoring biofuel feedstocks and conversion.

Biomedical Research & Diagnostics
Determining biomolecular structures, identifying and quantifying metabolites, and studying molecular interactions.

Textiles
Determining biomolecular structures, identifying and quantifying metabolites, and studying molecular interactions.

Environmental Science
Identifying and quantifying pollutants and characterizing organic matter in soil and water.
Semiconductors & Electronics
Analyzing purity and structure of materials used in manufacturing and packaging.
Complementary Techniques
- FTIR: Rapid functional group identification with minimal prep; ideal for fast screening but less detailed for complex structures.
- GC‑MS: Separates volatile compounds, provides molecular weight and fragmentation; highly sensitive but limited for isomers and detailed connectivity.
- ICP‑MS: Ultrasensitive elemental/isotopic detection for metals; complements NMR by providing elemental data instead of molecular structures.
- LC‑MS: Analyzes non‑volatile or labile biomolecules; delivers mass precision and sensitivity but less stereochemical insight.
Fourier Transform Infrared Spectroscopy (FTIR)
Rapid, non-destructive molecular fingerprinting across materials. Explore
Gas Chromatography-Mass Spectrometry (GC-MS)
Identifies and quantifies small organic molecules in mixtures. Explore
Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
Measures trace elements with high accuracy. Explore
Why Choose Covalent for Your NMR Needs?
At Covalent, we offer a full suite of NMR probes for both liquid and solid-state analyses, including specialized probes for challenging applications such as limited sample sizes, macromolecule diffusion, and advanced solid-state NMR. Complimentary deuterated solvents (CDCl₃, D₂O, DMSO-d₆, CD₃CN) are provided, and we ensure fast turnaround for routine liquid NMR on common isotopes (^1H, ^13C, ^19F, ^31P).
Our established qNMR workflow uses certified Sigma-Aldrich TraceCERT® reference materials, and we have expertise in non-standard sample preparation, including air-free transfers and handling corrosive materials.
Frequently Asked Questions
Identifying the right test or analysis can be complex, but it doesn’t have to be complicated.
Here are some questions we are frequently asked.
What’s NMR?
NMR stands for nuclear magnetic resonance. It studies the magnetic spin of a specific nucleus and extract structural and dynamic information of the sample.
What’s Covalent’s NMR capability?
Covalent operates a JEOL 500MHz NMR spectrometer with 3 liquid NMR probes and 2 solid NMR probes. We do both standard 1D/2D NMR and advanced methods such as diffusion NMR, solid-state NMR etc.
What element/isotopes can NMR detect?
Routine NMR isotopes are proton (1H), carbon-13 (13C), fluorine-19 (19F), phosphorus-31 (31P). Other common NMR isotopes are lithium-6/7, (6/7Li), boron-10/11 (10/11B), nitrogen-15 (15N), aluminum-27 (27Al) and silicon-29 (29Si).
What’s the general sample requirement for NMR?
For liquid NMR, the sample needs to be soluble in a deuterated solvent. For solid NMR, fine powders are preferred. No paramagnetic material (e.g. iron oxide) is allowed.
Why are deuterated solvents used in solution NMR?
deuterated solvents are used to suppress proton signals from the regular solvent, which could overwhelm the NMR spectra and obscure the sample signal. Deuterated solvent also provides a lock signal for magnetic field stability and facilitates shimming for field homogeneity.
What information can I get from NMR?
NMR can provide information on molecular structure and dynamics. You can identify bond connectivity and stereochemistry in organic compounds, measure sample purity, study diffusion dynamics of small molecules, monitoring reaction or degradation kinetics, understand binding interactions between molecules etc.
What’s the NMR data output?
1D NMR is a plot of chemical shift vs signal intensity. The chemical shift values, peak splitting patterns and peak integration can be analyzed to elucidate structure and composition. 2D NMR is a contour map of signal intensity. The two axes are chemical shifts of two specific isotopes, and the contour intensity shows correlation between the isotopes.