What Is Ion Scattering Spectroscopy (ISS)?
Ion Scattering Spectroscopy (ISS) – sometimes also called Low-Energy Ion Scattering (LEIS) or Low-Energy Ion Scattering Spectroscopy (LEISS) – is a method used to determine the composition of the outermost atomic layer of solid materials when sensitivity matters and precision is key. The ISS process bombards a material with a focused beam of low-energy ions – in the range of a few electron volts to a few kilo-electron (keV) range. Then, we measure the energy of ions scattered back from the surface to provide a highly accurate representation of elemental composition – electrical bombardment at the microscopic level and measurement of the scattering signal.
Exceptional Surface
Sensitivity
ISS exclusively probes the outermost atomic layer, providing unparalleled accuracy for surface composition analysis where even a single atomic layer impacts material performance.
Non-Destructive
and Adaptable
When operated in static mode, ISS preserves the sample, allowing sequential measurements over time. Depth profiling is also possible for controlled layer-by-layer analysis.
Complementary
Integration
ISS can be combined with XPS, ToF-SIMS, or AFM in situ for comprehensive surface and near-surface characterization without exposing samples to ambient conditions.
Why Use ISS?
Ion Scattering Spectroscopy offers unparalleled sensitivity to the outermost atomic layer. While methods like XPS or EDS probe several surface atomic layers, ISS focuses exclusively on the very first nuclear layer. As a result, it delivers accurate surface composition information without interference from subsurface signals. When coatings are extremely thin, this level of precision is extremely important.
This exceptional surface specificity makes ISS very valuable in applications where surface chemistry impacts performance. In catalysis, for example, only the atoms at the immediate surface participate in chemical reactions, with atoms below the surface shielded from the interaction. Similarly, in semiconductor manufacturing, the chemical integrity of surface and interfacial layers plays a crucial role in device functionality and reliability.
Due to these capabilities, ISS analysis has found widespread adoption across various sectors where surface to surface interaction matters, including semiconductor fabrication, catalysis research, thin film development, corrosion studies, and advanced materials science. When operated in static mode, ISS is non-destructive, allowing for sequential measurements that monitor surface changes over time – think processes like annealing, chemical treatments, and other surface modifications. If you want to intentionally go deeper, however, you can operate ISS in the same spot for an extended period and strip away layers of material, enabling the creation of depth profiles of elements. We can tell you with extreme accuracy how many licks it takes to get to the center of this (very small) Tootsie Pop.
Surface Composition Precision
Provides accurate elemental analysis of the top 1–2 monolayers for thin films, catalysts, and semiconductors.
Wide Application Across Industries
Ideal for semiconductors, catalysis, thin-film development, materials science, and corrosion studies.
Minimal Sample Preparation
Requires little to no preparation, enabling fast, high-sensitivity surface analysis while preserving samples.
Working Principle
Ion Scattering Spectroscopy operates on the principle of binary collision physics — basically, we see what happens when we crash things into each other. The basic principle of ISS is that a beam of low-energy ions hits a sample surface, with some ions backscattering at different energies depending on the mass of surface atoms. The balls on our pool table are made of different material, and how they bounce when our cue ball strikes them can be measured, telling us what each is made of.
However, the extreme surface sensitivity of ISS is due to two factors: the low penetration depth of the low-energy ions and the high neutralization probability of ions that penetrate beyond the topmost layer. ISS is uniquely capable of analyzing accurate surface composition because only ions scattered from the outermost atomic layer contribute to the detected signal. In short, we tap each cue ball very lightly and measure each reaction very carefully to disrupt the table as little as possible.
Equipment Used for ISS:
ThermoFisher Scientific Nexsa G2
- Ion beam of He/Ar/Ne.
- Energy range 500 to 2000eV.
- Internal reference of gold for primary energy calibration.
- Spot Size: 1 mm.
- Tilt option.
- Heating option.

Key Differentiators
At Covalent, we use the Nexsa G2 Surface Analysis System to conduct ISS measurements. This versatile instrument uniquely integrates ISS with X-ray Photoelectron Spectroscopy (XPS) and Ultraviolet Photoelectron Spectroscopy (UPS) within a single analysis chamber. This in situ capability allows for the sequential application of these surface-sensitive techniques on the same sample area without exposure to ambient air, generating a complementary dataset.
This type of approach is particularly critical for air-sensitive investigations, including research into catalytic properties, oxidation mechanisms, and temperature-dependent surface reactions, where maintaining surface integrity is imperative. In short, many surfaces react to ambient air and we can be sure that the results we observe are truly a result of the composition of your material, not your material exposed to the atmosphere.
Strengths
- Surface sensitivity: Exceptional sensitivity to the outermost atomic layers (typically top 1 to 2 monolayers).
- Elemental specificity: Capable of detecting all elements (except hydrogen and helium) based on their mass.
- Quantitative capability: Allows semi-quantitative to quantitative analysis of surface composition.
- Minimal sample preparation: Generally requires little to no special sample preparation.
Limitations
- Limited chemical state information: Does not provide direct insight into chemical bonding, states, or oxidation states.
- Can be destructive: Depth profiling involves sputtering, which is destructive to the sample.
- Complex interpretation: Data can require complex modeling or reference data for accurate interpretation.
- Surface contamination sensitivity: Very sensitive to adventitious carbon and other surface contaminants, which can obscure true surface composition.

Unsure Whether ISS Is Right for You?
Get monolayer‑level answers without exposing samples to air.
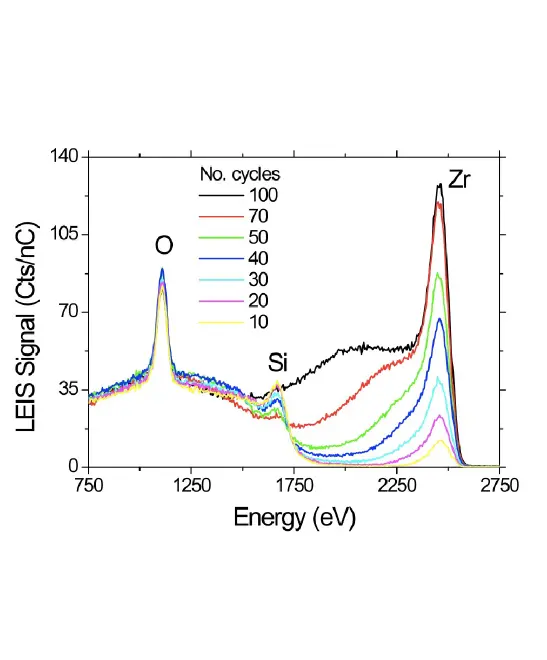
Sample Information
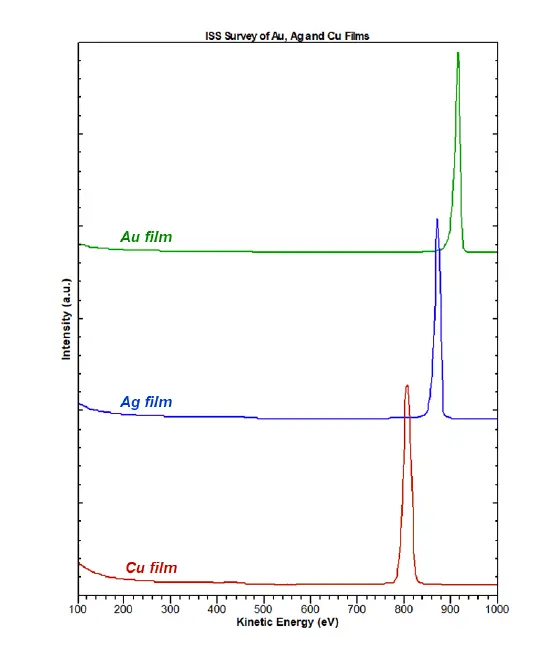
Overlay of ISS measurements taken on three different film species as a function of the kinetic energy of scattered ions. The results demonstrate peak separation for Copper (Cu), Silver (Ag), and Gold (Au).
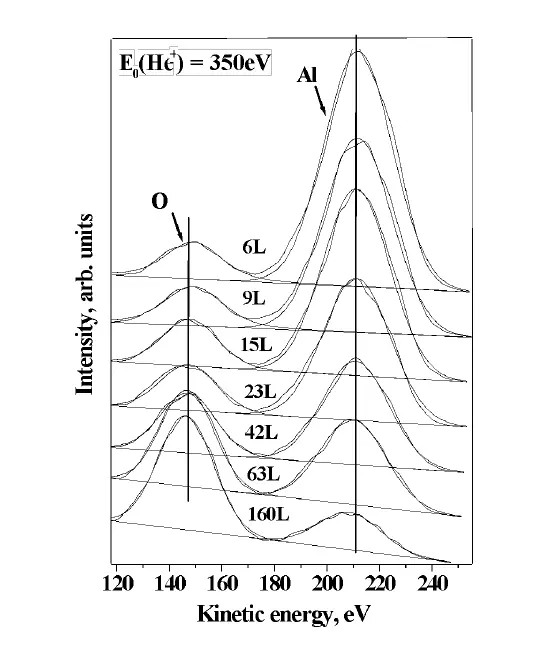
ISS spectra measured with E0(He+)=350eV on the Al surface as a function of exposure to oxygen. The increase in the oxygen peak comes together with the decrease in the aluminum peak, demonstrating the surface sensitivity of the technique.
What we accept:
Covalent’s experts help make sure that the samples you’re providing are a good match for the analysis we’re performing. Sample requirements for ISS include:
- Solid phase.
- Must be stable under high vacuum.
- Maximum Thickness: 20 mm.
- Maximum Lateral Dimensions: 60 mm x 60 mm.
- For better quantification, the sample should be as smooth as possible.
Use Cases
Ion Scattering Spectroscopy excels in applications where sensitivity to the upper surface layer is necessary, including:
Thin Film Technology
ISS analyzes the composition of the outermost layer of thin films, crucial for applications like displays, solar cells, and protective coatings. This is one of the few methods to measure monolayer film and determine full/partial coverage of the surface.

Catalysis Research
Surface composition directly determines catalytic performance. When you need to measure the efficiency and effectiveness of a catalyst that is intended to facilitate a chemical reaction, ISS testing helps researchers better understand catalyst surface composition before, after, and during reactions.
Semiconductor
ISS analyzes wafer contamination, interfacial composition in multilayer devices, and surface modification processes.

Materials Science
ISS can monitor surface segregation, alloying behavior, and surface reactions.
Complementary Techniques
ISS works well with other analytical techniques and provides comprehensive surface and near-surface characterization. Other techniques would be used when we need extra depth, sensitivity, or correlation of material identification with topographic or physical properties.
- Atomic Force Microscopy (AFM): Combining ISS with AFM allows a correlation of surface composition with topography and physical properties. While ISS reveals what elements are present at the surface, AFM shows those surfaces’ physical structure and properties.
- Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS): ToF-SIMS offers higher sensitivity and can detect isotopes and molecular fragments, while ISS provides straightforward quantification of elemental composition. ISS is generally less destructive than SIMS and focuses exclusively on the outermost layer, while SIMS can detect trace contaminants below the detection limit of ISS.
- X-ray Photoelectron Spectroscopy (XPS): While ISS provides an elemental composition of the outermost atomic layer, XPS offers chemical state information and composition analysis from several nanometers of depth. Together, they offer both extreme surface and near-surface information.
Atomic Force Microscopy (AFM)
Maps nanoscale topography and material properties with a sharp probe. Explore
Time of Flight Secondary Ion Mass Spectroscopy (ToF-SIMS)
Ultra-sensitive surface analysis with chemical imaging & depth profiling. Explore
X-ray Photoelectron Spectroscopy (XPS)
Measures surface elemental composition and chemical states. Explore
Why Choose Covalent for Your ISS Needs?
At Covalent, we offer distinct advantages for Ion Scattering Spectroscopy analysis.
Unparalleled Technical Excellence:
- Advanced Instrumentation: Our ThermoFisher Scientific Nexsa G2 spectrometer is state-of-the-art in ISS capabilities, offering expert-level sensitivity and resolution.
- Expert Team: Our scientists have extensive experience in surface analysis techniques and specialize in solving complex materials challenges.
- Integrated Analysis: We can combine ISS with complementary techniques (XPS, SIMS, AFM) for comprehensive surface characterization within a single project.
Top-Level Service Advantages:
- Rapid Turnaround: Standard ISS analysis available with a 48-hour turnaround time, with expedited service options.
- Collaborative Approach: Our scientists work directly with clients to design experiments that address specific technical questions.
- Comprehensive Reporting: Detailed analysis reports include not only raw data but expert interpretation and actionable insights.
- Method Development: Custom ISS protocols can be developed for unique sample types or specific research questions.
Frequently Asked Questions
Identifying the right test can be complex, but it doesn’t have to be complicated.
Here are some questions we are frequently asked.
Can ISS detect all elements in the periodic table?
ISS can detect elements from nitrogen (Z=14) to uranium (Z=92).
Is ISS analysis destructive to the sample?
In static mode (low ion dose), ISS is essentially non-destructive. However, a longer measurement time will destroy the sample. Sometimes, the destruction is the point of the measurement, other times it is a necessary consequence of the measurement being performed.
What information does ISS provide that XPS cannot?
While XPS provides chemical state information from several nanometer depths, ISS gives the accurate elemental composition of only the outermost atomic layer. Sometimes the surface needs to be measured and the deeper layers are either irrelevant or specifically different (here you’d use ISS) and sometimes depth matters (choose XPS).
What is the typical turnaround time for ISS analysis at Covalent?
Standard ISS analysis is typically completed within five business days from sample receipt. Expedited service options are available for time-sensitive projects, potentially providing results within 24 hours.