What Is Inductively Coupled Plasma Mass Spectrometry (ICP-MS)?
Inductively Coupled Plasma Mass Spectrometry, also known as ICP-MS, is an elemental and isotopic analysis method that uses a high-temperature radio-frequency plasma to ionize materials. These ions are then separated and quantified by a mass spectrometer, providing both concentration and isotopic information at ultra-trace levels.
ICP-MS is commonly used to measure trace metals in aqueous solutions, and can achieve sensitivities that can reach up to parts-per-quadrillion. This technique can also maintain linear responses across several orders of magnitude and deliver simultaneous multi-element detection.
Ultra-trace Detection
Measures elements at ppt to ppq levels for highly sensitive analysis.
Isotopic Precision
Resolves isotopic ratios for tracer studies, contamination source analysis, and research.
Wide Material Range
Works with liquids, solids, gases, and complex matrices across industries.
Why Use ICP-MS?
ICP-MS is the go-to choice when the task demands the most precise elemental insights. It excels in trace and ultra-trace elemental analysis, resolving concentrations that other techniques find difficult to detect.
The technique is applied across a broad and critical range of applications, and scientists depend on it for trace metal detection, elemental impurity analysis, and isotopic ratio measurement. These measurements form the backbone of decision-making in research, production, and compliance testing.
Because of this versatility, ICP‑MS has become integral across industries. It protects purity in semiconductor workflows, verifies compliance in pharmaceutical development, supports biomedical research, and enables monitoring in environmental, regulatory, energy, and forensic settings.
At the lowest detectable levels, where precision is non‑negotiable, ICP‑MS delivers clarity.
Triple-Quadrupole ICP-MS
Removes interferences, enabling accurate sub-ppb quantification.
Laser Ablation Capability
Analyzes solids directly with micrometer-scale mapping and depth profiling.
Flexible Workflows
Supports automation, chromatography, and high-throughput sample processing.
Working Principle
Any technique begins with sample preparation, and ICP-MS is no different. In ICP-MS, the solid samples are digested, often with the help of acid, and the liquids are diluted. The prepared sample is then pumped through a nebulizer, which sprays it into a fine mist.
The mist is then swept into an argon plasma (typically operating at thousands of degrees Celsius). Rapidly, the plasma dries, decomposes the sample, and ionizes the constituent elements, forming +1 ions. These ions are directed through a sampling interface toward the mass analyzer. In a quadrupole system, they are separated by their mass-to-charge ratio (m/z) and counted by an electron multiplier.
At Covalent, this workflow lets us quantify elements across wide concentration ranges and deliver validated, robust reports for regulated and industrial applications.
Equipment Used for ICP-MS:
At Covalent, we pair the Thermo Scientific iCAP TQ ICP‑MS with the Teledyne Photon Machines IRIDIA Laser Ablation system to cover both traditional liquid workflows and advanced solid‑sample analysis without compromise.
Thermo Scientific iCAP TQ ICP‑MS
Some key features include:
- Triple‑quadrupole interference removal: TQ technology with a Collision/Reaction Cell to strip away complex matrix interferences, enabling confident sub‑ppt elemental quantification.
- High sensitivity with routine usability: Provides the lowest detection limits in Thermo’s benchtop ICP‑MS line while still supporting simple method development through Qtegra™ ISDS software and Reaction Finder tools.
- Consistency at scale: Engineered for uptime and repeatability, minimizing downtime and ensuring consistent, accurate results even over large sample batches.
- Flexible coupling with advanced sampling devices: Compatible with automation, chromatography, and laser ablation, expanding analysis modes beyond solution nebulization.
- Direct solid analysis with IRIDIA laser ablation: Fast washout (<1 ms), homogeneous energy delivery, and variable spot sizes allow high‑resolution elemental mapping and depth profiling of solid materials without full digestion.

Key Differentiators
Strengths
- Exceptional Sensitivity.
- Isotopic Discrimination.
- Wide Dynamic Range.
- Material Compatibility.
- Spatial Resolution and Depth Profiling.
- Flexible Workflows.
Limitations
- Most samples need conversion into a solution (excluding laser ablation); prep can be difficult depending on element solubility and stability.
- Accuracy requires calibration with matrix-matched standards.
- Isotopic measurements can be affected by polyatomic interferences.
- Limited to elemental analysis; provides no molecular or bonding information.
- Surface specificity is limited; depth profiling only possible with laser ablation.
- Technique depends on careful sample prep and interference management.

Unsure Whether ICP‑MS Is Right for Your Sample?
Learn how ICP‑MS can help you make confident material decisions.
Sample Information
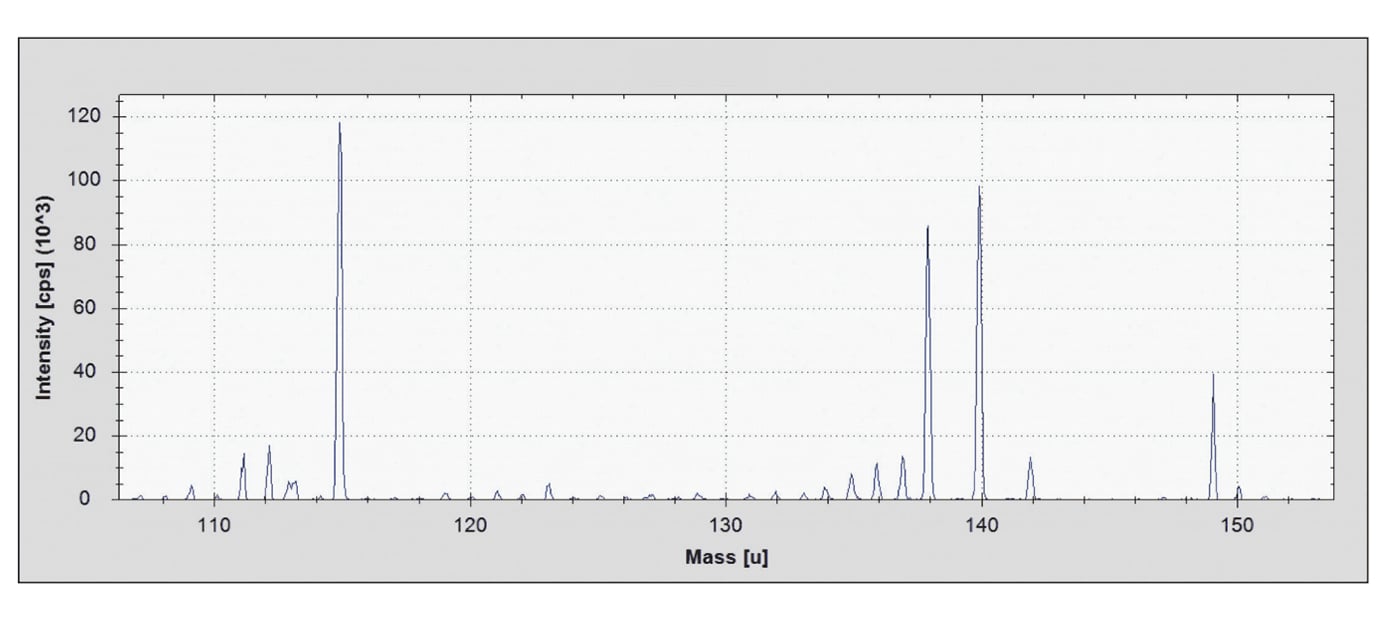
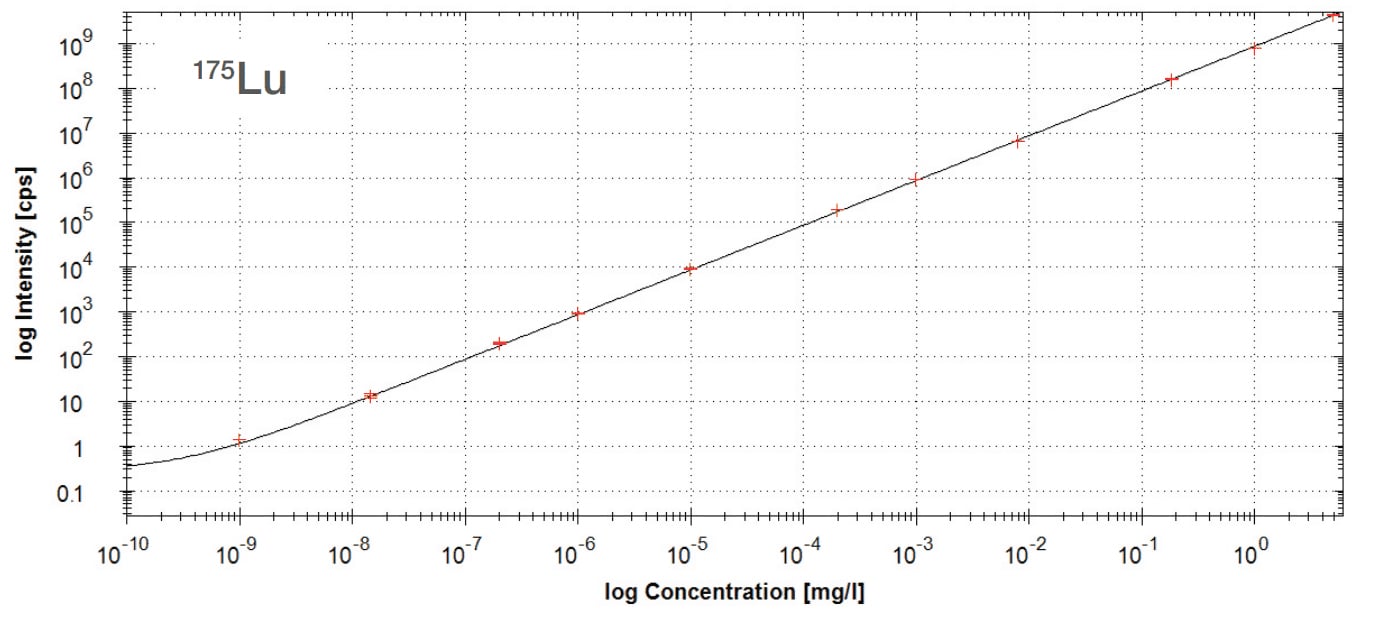
Each ICP-MS run produces spectra and calibration curves that highlight both elements detected and their concentrations (as shown below). However, this data can be presented in multiple forms depending on the analysis:
Mass Spectrum: Here’s a high-resolution scan across the 105–155 amu range. Each peak represents a different element or isotope in the sample. Even at ultra-low concentrations, ICP-MS can pull out a clear signal with very little background noise; this acts as the “fingerprint” of the elemental makeup.
Calibration Curve: The image below shows a quantification curve for ^175Lu. The straight-line relationship across more than 10 orders of magnitude demonstrates just how wide the dynamic range is. Simply put, ICP-MS can measure incredibly tiny amounts and still stay accurate all the way up to much higher concentrations
At Covalent, we take this raw data and transform it into clear, actionable reports. Our clients receive:
- A list of all detected elements (and isotopes, if part of the study).
- Concentration values in ppb or ppm, corrected for digestion/dilution.
- Contextual interpretation from our experts, so the numbers are meaningful for your application or regulatory question.
What we accept:
When it comes to sample prep for ICP-MS, getting a gas, liquid, or solid sample ready for plasma ionization is the difference between accurate, actionable insights and inconclusive data.
- Acceptable forms: Gases, liquids, and solids can be measured once prepared for the plasma.
- Small sample quantities: Only a few milligrams are often enough when properly digested or diluted.
- Key properties: Volatility, solubility, and stability of the sample guide the preparation route.
- Special considerations: Organics, ceramics, and refractory materials require tailored digestion before analysis.
Covalent reviews every sample case by case, tailoring preparation so our clients can count on accurate, high‑sensitivity measurements.
Use Cases
ICP-MS delivers critical elemental analysis across a broad spectrum of industries and research fields. Some key applications include:

Mining & Geology
ICP-MS detects platinum group metals like Pt, Pd, Rh, and Ir at sub-ppb levels in rocks and ores. This allows geologists to accurately assess ore value and plan extraction strategies that would be missed with less sensitive methods.

Oil & Petrochemical Refining
Refineries use ICP-MS to monitor trace elements such as Ni, V, and rare earth metals in fuel oils and petrochemical streams. Even minimal concentrations can impact catalyst lifetimes, process efficiency, and regulatory compliance, making precise detection critical.

Pharmaceuticals
ICP-MS ensures regulatory compliance (e.g., ICH Q3D) by measuring heavy metals like arsenic, cadmium, mercury, and lead well below required thresholds. This protects patient safety while supporting quality assurance in drug development.

Environmental Monitoring
Communities rely on ICP-MS for early detection of toxic elements, such as inorganic arsenic in groundwater. The technique distinguishes harmful inorganic forms from safe organic species, enabling rapid protective actions.
Semiconductor & Advanced Materials
Sub‑ppt detection of alkali and transition metals is essential for high-purity wafers and chemicals in chip fabrication. ICP-MS reveals trace contaminants that can reduce yields or damage device performance. Laser ablation capabilities further allow high-resolution mapping of solid samples and precise trace element analysis in challenging matrices.
Complementary Techniques
- Combustion Analysis and Inert Gas Fusion: Used to measure atmospherics like C, H, N, S, and O, which ICP-MS cannot quantify effectively. These are bulk, destructive techniques, complementary for elemental analysis of gases or solids.
- GD-MS: Ideal for ultra-trace bulk analysis in solids. Complements ICP-MS when extreme sensitivity for metals in difficult matrices is required.
- ICP-OES: Shares a similar plasma-based measurement principle but uses optical emission spectroscopy instead of mass spectrometry. While ICP-OES is less sensitive than ICP-MS, it can handle slightly more complex matrices. ICP-MS provides the added advantage of isotopic analysis, which ICP-OES cannot.
- Ion Chromatography: Sensitive to halides and small organic acids, which are outside the typical measurement scope of ICP-MS.
- SEM-EDS: Offers lower sensitivity (~0.1 wt%) but can be non-destructive. SEM-EDS can measure elements such as carbon, oxygen, nitrogen, and halides that are difficult for ICP-MS, though light elements like Li and Be are more easily quantified with ICP-MS.
- XPS: Surface-sensitive and non-destructive, XPS measures elemental composition and provides molecular or bonding information. Sensitivity is lower (~0.1 at%), but it gives chemical context that ICP-MS cannot.
- XRF: Provides rapid, non-destructive chemical or elemental analysis of solids with minimal sample preparation. Sensitivity is lower than ICP-MS, and accuracy can be affected by sample homogeneity.
Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
Quantifies multiple elements at very low concentrations. Explore
Scanning Electron Microscopy (SEM)
Images surface topography and composition with electrons. Explore
X-ray Photoelectron Spectroscopy (XPS)
Measures surface elemental composition and chemical states. Explore
Why Choose Covalent for Your ICP-MS Needs?
At Covalent, we ensure every sample is reviewed by experts who understand the preparation, matrix effects, and interferences that can make or break the ICP-MS measurements. This, in turn, helps us deliver reliable, accurate data, faster turnaround, and the technical clarity our clients need to move forward.
Frequently Asked Questions
Identifying the right test can be complex, but it doesn’t have to be complicated.
Here are some questions we are frequently asked.
Can ICP-MS be used for isotopic ratio analysis?
Yes. ICP‑MS is routinely used to measure isotopic ratios with high precision.
What is the difference between quadrupole and ToF ICP-MS?
A quadrupole mass spectrometer filters ions so that only one mass‑to‑charge ratio (m/z) is measured at a time. In contrast, a ToF analyzer separates ions by their velocity after acceleration through an electric field, allowing detection of a wide range of m/z values simultaneously.
How do I prepare high-salt samples for ICP-MS?
High‑salt samples are best diluted significantly before analysis to reduce matrix effects and ensure compatibility with the plasma.
How does laser ablation ICP-MS work for solid samples?
A focused laser beam ablates tiny particles from the surface of a solid sample. These particles are transported to the RF plasma by a carrier gas, at which point they are ionized, separated, and measured in the same way as a solution sample in ICP‑MS.